Properties
- Higher boiling points than alkanes
Aldehydes and ketones have higher boiling points than alkanes with a similar formula mass due to the presence of Van der Waals and permanent dipole-dipole forces between the polar carbonyl groups.
- Lower boiling points than alcohols
Alcohols (with a similar formula mass) will have higher boiling points as hydrogen bonds can form between alcohol molecules.
- Short chains are soluble in water
Short chain aldehydes and ketones are soluble in water as they can form hydrogen bonds with water molecules – the C=O bond is polar.
Oxidation
Using acidified potassium dichromate (VI) aldehydes can be oxidised further from primary alcohols to form carboxylic acids.

Reduction
Aldehydes and ketones can be reduced using the reducing agent: sodium tetrahydridoborate (III), NaBH4 in aqueous solution.
Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohols (opposite of oxidation).
You need to know the nucleophilic addition mechanisms for the reduction of aldehydes and ketones using NaBH4 (represented as H–).
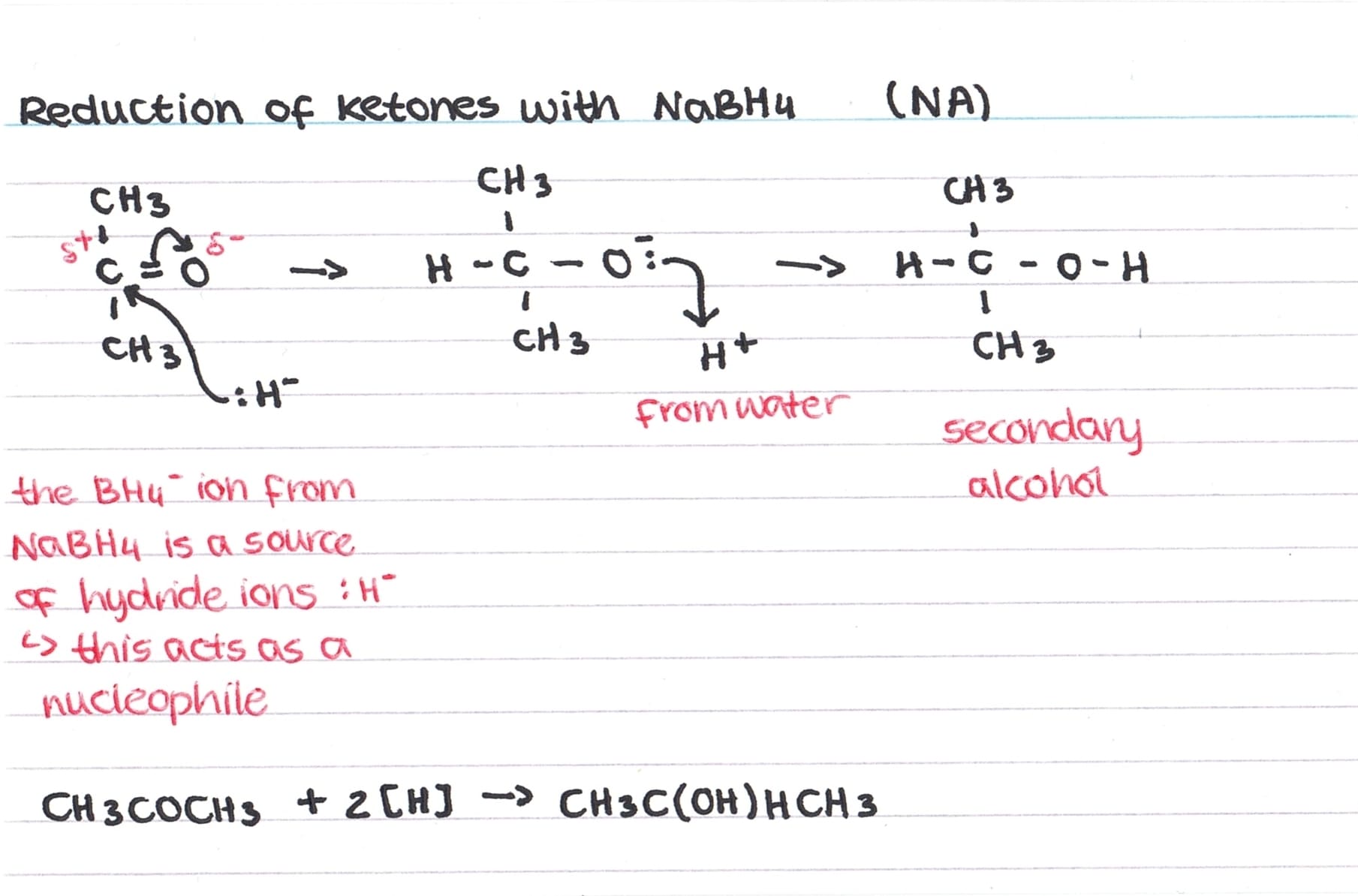
The nucleophillic addition mechanism for reducing a ketone can be seen above – the same method is followed for an aldehyde.
Testing for Aldehydes and Ketones
The following mild oxidising agents can be used to distinguish between aldehydes and ketones (aldehydes can be further oxidised but ketones cannot).
Fehling’s Solution
- Warm unknown solution with Fehling’s solution (blue) in a water bath
- If a ketone is present, solution remains blue
- If an aldehyde is present, orange-red precipitate forms
- Cu2+ ions in Fehling’s are reduced by the aldehyde to form Cu2O
Cu2+ + e– -> Cu+

Tollens’ Reagent
- Warm unknown solution with Tollens’ in a water bath
- If a ketone is present, solution remains colourless
- If an aldehyde is present, silver mirror forms on sides of test tube
- Ag+ ions in Tollens’ are reduced to form silver
Ag+ + e– -> Ag

The photos above show positive tests for an aldehyde.
Formation of Hydroxynitriles using HCN
We need to be able to write overall equations for the formation of hydroxynitriles using hydrogen cyanide, HCN. Examples can be seen below:
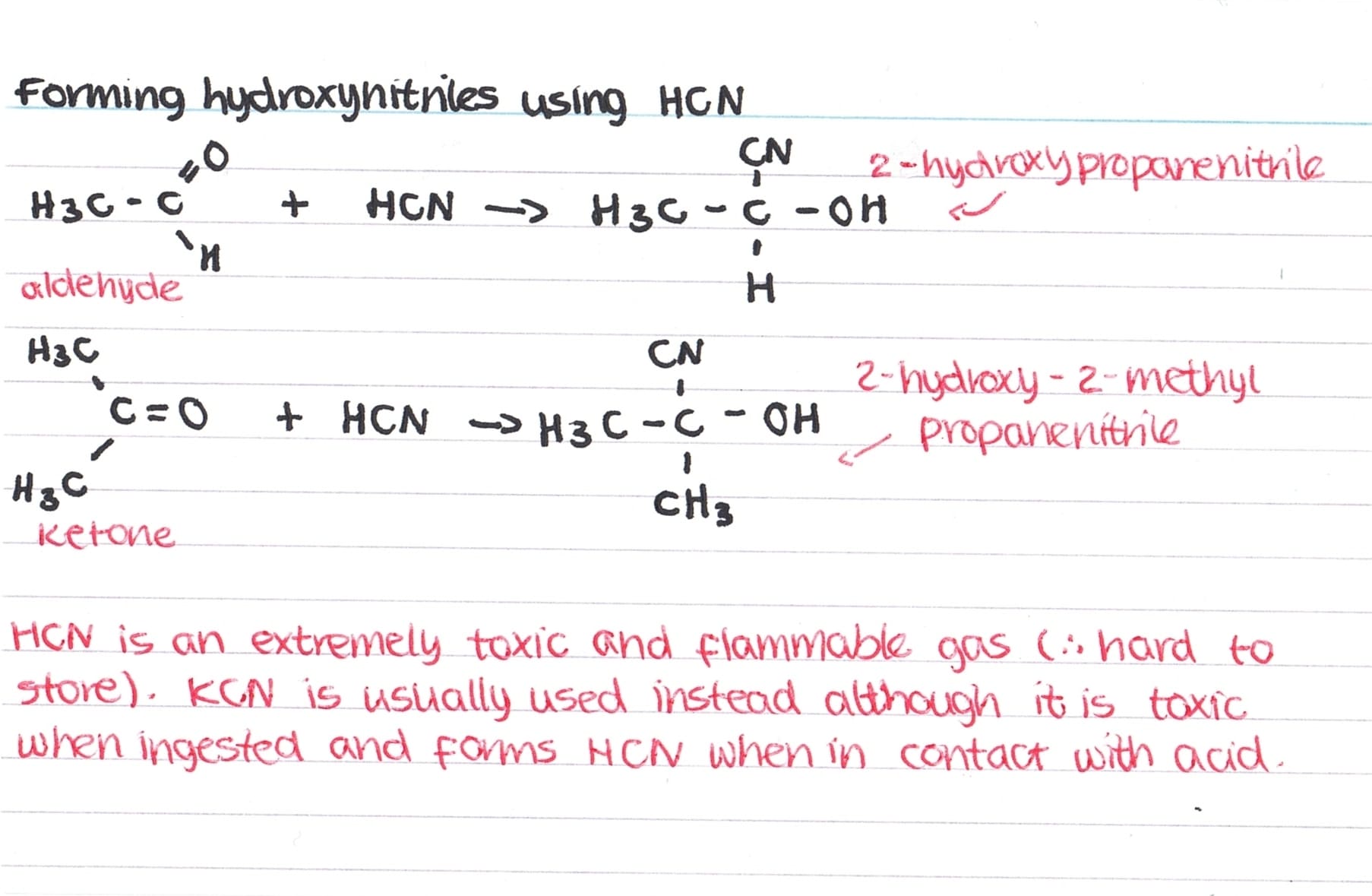
As HCN is toxic and flammable, KCN is usually used instead. When hydroxynitriles are named, the carbon attached to the nitrogen is taken as carbon 1 (the OH is a side group). In addition, as HCN is a weak acid it only partially dissociates so the concentration of cyanide ions is low, therefore, it is better to use KCN (provides higher concentration of CN–).
Nucleophilic Addition of KCN to form Hydroxynitriles
For the formation of hydroxynitriles using KCN, we need to be able to outline the nucleophilic addition mechanism.
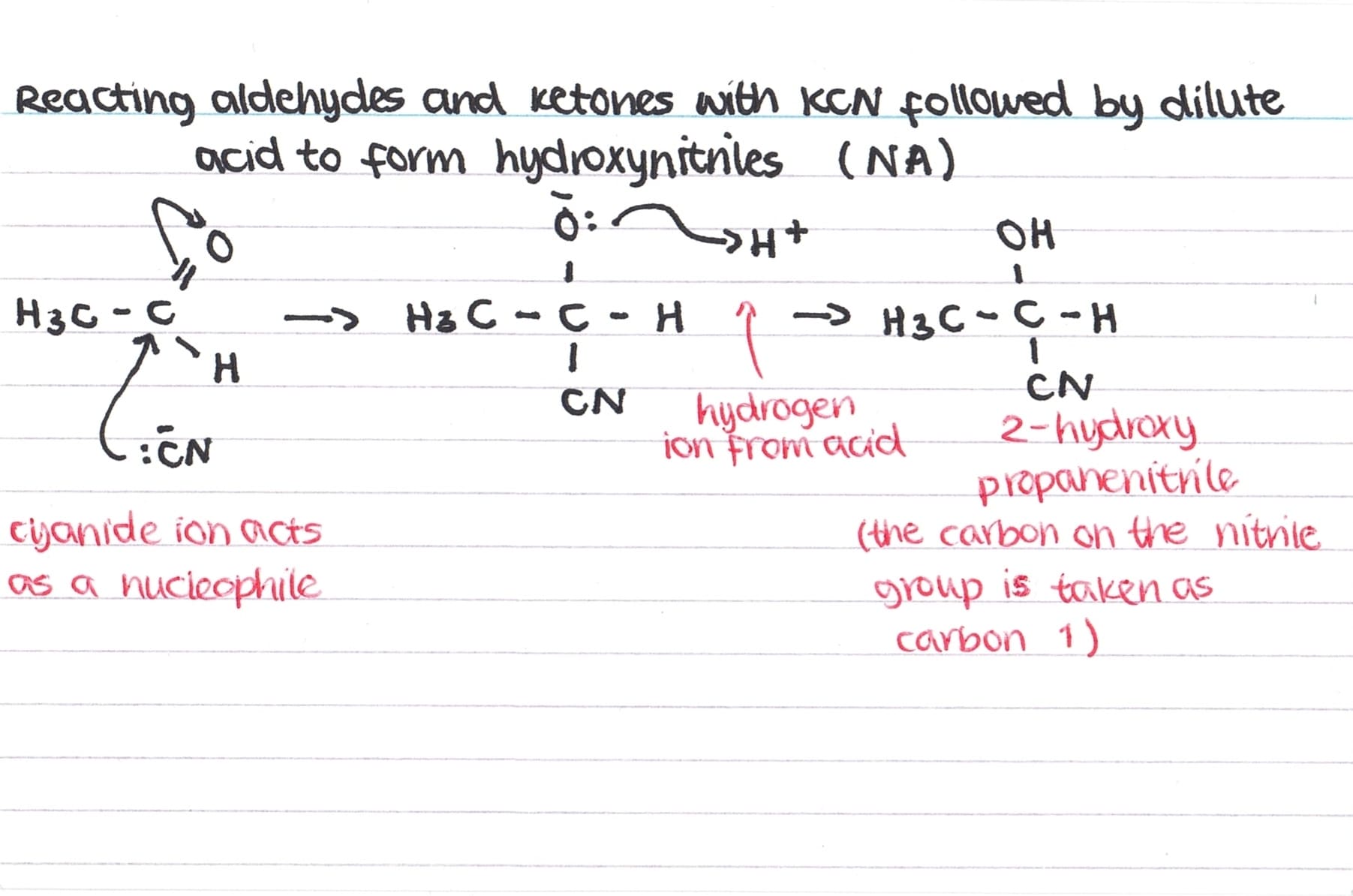
As the carbonyl bond is planar, there is an equal chance of it being attacked from above or below by the cyanide ion – this results in the formation of enantiomers. However, as there is an equal chance of each enantiomer forming, a racemic mixture is formed so overall the solution is optically inactive.
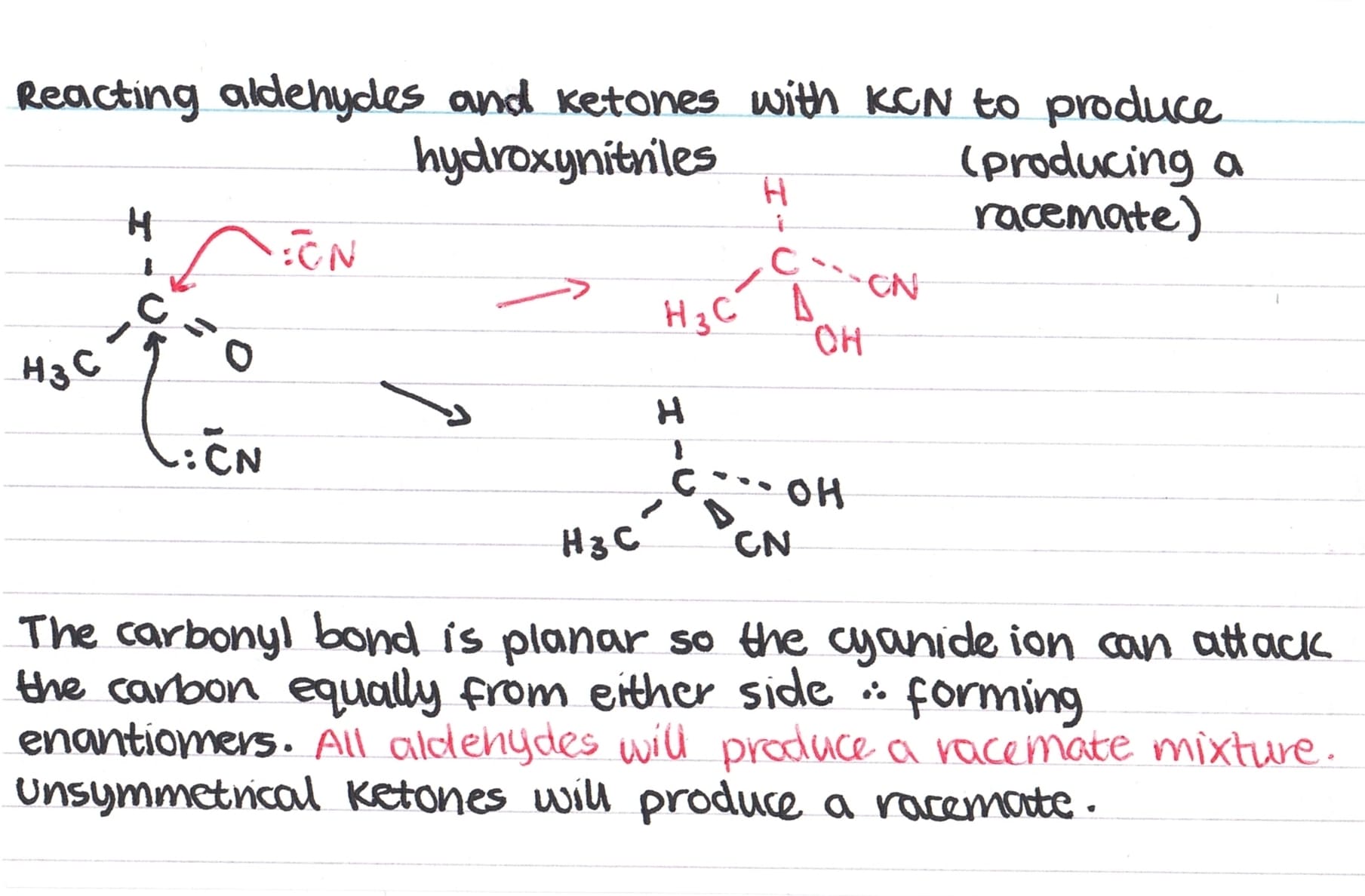
All aldehydes will form a racemic mixture but only unsymmetrical ketones will also form a racemic mixture.
